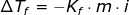"Published in Newark, California, USA"
A 0.2 m aqueous solution of KCl freezes at -0.680°C. What is the osmotic pressure at 0°C?
Solution:
Since the freezing point of KCl solution is given, then we have to use the Freezing Point Depression Formula in order to get the molality of KCl solution as follows
If Kf of water is 1.86°C/m and i for KCl is 2, then the molality of a solution is
which is close to 0.2 m. Next, we need to convert molality into molarity as follows
where ρ is the density of a solution. If the given molality of KCl solution is 0.2 m, then the %KCl by weight is
By looking at the Properties of KCl Solutions, the density of 1.5% KCl is 1009.5 kg/m3 or 1.0095 kg/L at 20°C which is almost equal to the density of pure water. If you decrease the temperature, then its density will decrease also. Let's approximate that the density of 1.5% KCl solution is 1kg/L.
Hence, the molarity of KCl solution is
The osmotic pressure of any solution is given by the equation
where
Π = is the osmotic pressure of a solution
M = is the molarity of a solution
R = is the universal gas law constant
T = is the absolute temperature of a solution
i = is the dimensionless Van't Hoff factor
The universal gas law constant for gmole, atm, liters, and K is
 .
.Therefore, the osmotic pressure of KCl solution at 0°C is