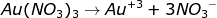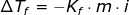Category: Chemical Engineering Math
"Published in Newark, California, USA"
An aqueous solution of gold nitrate is electrolyzed with a current of 0.555 amperes until 1.32 g of Au has been deposited on the cathode. If the atomic weight of Au is 197, determine the duration of the electrolysis.
Solution:
During the electrolysis process, gold (III) nitrate solution is ionized completely as follows
With the presence of electric current, gold is deposited on the cathode as follows
By using Faraday's Law of Electrolysis, we can solve for the time or duration of electrolysis of gold as follows
where:
W = weight of a metal formed during electrolysis
EW = equivalence weight of a metal
I = current or rate of flow of electrons
t = time or duration of electrolysis
Therefore, the time or duration of electrolysis of gold is
or
Note: In electroplating industries and jewelry industries, they are using the principles of electrolysis in coating a metal with another metal. A metal to be coated acts as a cathode and another metal used in coating acts as an anode in electrolysis.

This website will show the principles of solving Math problems in Arithmetic, Algebra, Plane Geometry, Solid Geometry, Analytic Geometry, Trigonometry, Differential Calculus, Integral Calculus, Statistics, Differential Equations, Physics, Mechanics, Strength of Materials, and Chemical Engineering Math that we are using anywhere in everyday life. This website is also about the derivation of common formulas and equations. (Founded on September 28, 2012 in Newark, California, USA)
Thursday, June 18, 2015
Wednesday, June 17, 2015
Osmotic Pressure Problems, 2
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 0.2 m aqueous solution of KCl freezes at -0.680°C. What is the osmotic pressure at 0°C?
Solution:
Since the freezing point of KCl solution is given, then we have to use the Freezing Point Depression Formula in order to get the molality of KCl solution as follows
If Kf of water is 1.86°C/m and i for KCl is 2, then the molality of a solution is
which is close to 0.2 m. Next, we need to convert molality into molarity as follows
where ρ is the density of a solution. If the given molality of KCl solution is 0.2 m, then the %KCl by weight is
By looking at the Properties of KCl Solutions, the density of 1.5% KCl is 1009.5 kg/m3 or 1.0095 kg/L at 20°C which is almost equal to the density of pure water. If you decrease the temperature, then its density will decrease also. Let's approximate that the density of 1.5% KCl solution is 1kg/L.
Hence, the molarity of KCl solution is
The osmotic pressure of any solution is given by the equation
where
Π = is the osmotic pressure of a solution
M = is the molarity of a solution
R = is the universal gas law constant
T = is the absolute temperature of a solution
i = is the dimensionless Van't Hoff factor
The universal gas law constant for gmole, atm, liters, and K is .
.
Therefore, the osmotic pressure of KCl solution at 0°C is
"Published in Newark, California, USA"
A 0.2 m aqueous solution of KCl freezes at -0.680°C. What is the osmotic pressure at 0°C?
Solution:
Since the freezing point of KCl solution is given, then we have to use the Freezing Point Depression Formula in order to get the molality of KCl solution as follows
If Kf of water is 1.86°C/m and i for KCl is 2, then the molality of a solution is
which is close to 0.2 m. Next, we need to convert molality into molarity as follows
where ρ is the density of a solution. If the given molality of KCl solution is 0.2 m, then the %KCl by weight is
By looking at the Properties of KCl Solutions, the density of 1.5% KCl is 1009.5 kg/m3 or 1.0095 kg/L at 20°C which is almost equal to the density of pure water. If you decrease the temperature, then its density will decrease also. Let's approximate that the density of 1.5% KCl solution is 1kg/L.
Hence, the molarity of KCl solution is
The osmotic pressure of any solution is given by the equation
where
Π = is the osmotic pressure of a solution
M = is the molarity of a solution
R = is the universal gas law constant
T = is the absolute temperature of a solution
i = is the dimensionless Van't Hoff factor
The universal gas law constant for gmole, atm, liters, and K is
 .
.Therefore, the osmotic pressure of KCl solution at 0°C is
Tuesday, June 16, 2015
Empirical and Molecular Formula Problems, 8
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A mixture of 0.660 grams of camphor and 0.050 grams of an organic solute freezes at 157°C. If the solute contains 10.5% H by weight, determine the molecular formula of the solute if the freezing point of camphor is 178.4°C and the value of Kf is 38.
Solution:
The freezing point constant is defined as the number of degrees the freezing point will be lowered per mole of solute per 1000 g or 1 kg of solvent present. This can be written as
where m is the molality of a solution.
The freezing point depression is defined as the product of the freezing point constant of a solvent and the molality of a solution.
Hence, the molality of a solution is
If the weight of a solvent which is camphor is given, then we can solve for the moles of an organic solute as follows
The molecular weight of an organic solute is
In 1 gmole of an organic solute, if the percent of H is 10.5% by weight, then the weight of H is
and the weight of C is
The number of moles of H is
and the number of moles of C is
In order to get the number of atoms of C and H, we need to divide them1. with the smallest number of moles. In this case, C has a number of smallest moles.
The number of H atoms is
and the number of C atoms is
Hence, the empirical formula of an organic solute is CH1.4.
Since CH1.4 cannot be accepted as a molecule, then we have to multiply each number of atoms by 10 in order to eliminate the decimal number at H atoms.
Therefore, the molecular formula of an organic solute is C10H14 which is an aromatic compound.
"Published in Newark, California, USA"
A mixture of 0.660 grams of camphor and 0.050 grams of an organic solute freezes at 157°C. If the solute contains 10.5% H by weight, determine the molecular formula of the solute if the freezing point of camphor is 178.4°C and the value of Kf is 38.
Solution:
The freezing point constant is defined as the number of degrees the freezing point will be lowered per mole of solute per 1000 g or 1 kg of solvent present. This can be written as
where m is the molality of a solution.
The freezing point depression is defined as the product of the freezing point constant of a solvent and the molality of a solution.
Hence, the molality of a solution is
If the weight of a solvent which is camphor is given, then we can solve for the moles of an organic solute as follows
The molecular weight of an organic solute is
In 1 gmole of an organic solute, if the percent of H is 10.5% by weight, then the weight of H is
and the weight of C is
The number of moles of H is
and the number of moles of C is
In order to get the number of atoms of C and H, we need to divide them1. with the smallest number of moles. In this case, C has a number of smallest moles.
The number of H atoms is
and the number of C atoms is
Hence, the empirical formula of an organic solute is CH1.4.
Since CH1.4 cannot be accepted as a molecule, then we have to multiply each number of atoms by 10 in order to eliminate the decimal number at H atoms.
Therefore, the molecular formula of an organic solute is C10H14 which is an aromatic compound.
Subscribe to:
Comments (Atom)