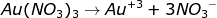Category: Chemical Engineering Math
"Published in Newark, California, USA"
An aqueous solution of gold nitrate is electrolyzed with a current of 0.555 amperes until 1.32 g of Au has been deposited on the cathode. If the atomic weight of Au is 197, determine the duration of the electrolysis.
Solution:
During the electrolysis process, gold (III) nitrate solution is ionized completely as follows
With the presence of electric current, gold is deposited on the cathode as follows
By using Faraday's Law of Electrolysis, we can solve for the time or duration of electrolysis of gold as follows
where:
W = weight of a metal formed during electrolysis
EW = equivalence weight of a metal
I = current or rate of flow of electrons
t = time or duration of electrolysis
Therefore, the time or duration of electrolysis of gold is
or
Note: In electroplating industries and jewelry industries, they are using the principles of electrolysis in coating a metal with another metal. A metal to be coated acts as a cathode and another metal used in coating acts as an anode in electrolysis.