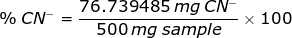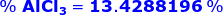Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 750.25 mg alloy of nickel was dissolved and treated to remove the impurities. The ammonium solution was treated with 50 mL of 0.1075 M KCN and the excess cyanide required 2.25 mL of 0.00925 M AgNO3. Determine the % Ni in the alloy.
Solution:
If nickel is dissolved in ammonium solution, then nickel is converted into nickel ion in order to remove the impurities. If KCN solution is added to nickel solution, then the chemical reaction is
From the chemical reaction above, we can solve for the weight of nickel as follows
Therefore, the percent of nickel in the alloy is
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 500 mg sample containing NaCN required 23.50 mL of 0.1255 M AgNO3 to obtain a permanent faint turbidity. Express the result of this analysis as %CN-.
Solution:
If AgNO3 is added to NaCN, then the chemical reaction is
From the chemical reaction above, the weight of CN- is
Therefore, the percent of CN- in a sample is
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 1.500 gram sample of impure AlCl3 was dissolved in water and treated with 45.32 mL of 0.1000 M AgNO3 using Mohr Method. Determine its purity.
Solution:
If AlCl3 solution is treated with AgNO3, then the chemical reaction is
From the chemical reaction above, the weight of pure AlCl3 is
Therefore, the percent of AlCl3 in a sample is