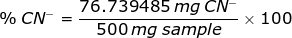Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 500 mg sample containing NaCN required 23.50 mL of 0.1255 M AgNO3 to obtain a permanent faint turbidity. Express the result of this analysis as %CN-.
Solution:
If AgNO3 is added to NaCN, then the chemical reaction is
From the chemical reaction above, the weight of CN- is
Therefore, the percent of CN- in a sample is