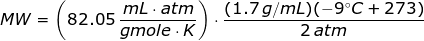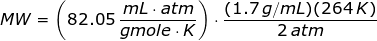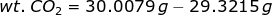Category: Chemical Engineering Math
"Published in Newark, California, USA"
At what temperature will the velocity of CO2 molecules equal the velocity of oxygen molecules at 0°C?
Solution:
The given word problem is about Kinetic Molecular Theory because it involves the velocity and temperature of gases. The velocity of a gas at a certain temperature is
where:
u = velocity of a gas
R = universal gas law constant
T = absolute temperature of a gas
M = molecular weight of a gas
Hence, the temperature of CO2 in K if its velocity is equal with O2 is
Therefore, the temperature of CO2 in °C is

This website will show the principles of solving Math problems in Arithmetic, Algebra, Plane Geometry, Solid Geometry, Analytic Geometry, Trigonometry, Differential Calculus, Integral Calculus, Statistics, Differential Equations, Physics, Mechanics, Strength of Materials, and Chemical Engineering Math that we are using anywhere in everyday life. This website is also about the derivation of common formulas and equations. (Founded on September 28, 2012 in Newark, California, USA)
Monday, June 15, 2015
Sunday, June 14, 2015
Empirical and Molecular Formula Problems, 7
Category: Chemical Engineering Math
"Published in Newark, California, USA"
The following data were obtained in the determination of a HC gas containing 92.3% C by weight at -9°C at various pressure:
Identify the gas.
Solution:
The given problem is about finding the molecular formula of a hydrocarbon gas using the principles of empirical and molecular formula. Since the given items are temperature, pressure, and density, then we have to use the Ideal Gas Law in order to get the molecular weight at different pressures and densities.
Let's consider the Ideal Gas Law equation:
but
and the above equation becomes
but
Hence, the Ideal Gas Law equation if the density is given is
where:
MW = molecular weight of unknown gas
ρ = is the density of unknown gas
R = universal gas law constant
T = absolute temperature of unknown gas
P = pressure of unknown gas
Looking at the given table above, if you get the average of the given pressures and densities, then the value of P is 2 atm and the value of ρ is 1.7 g/mL.
The molecular weight of the unknown gas is
In one mole of unknown gas, the weight of C is
and the weight of H is
The number of moles of C is
and the number of moles of H is
In order to get the number of atoms of C and H, we need to divide them with the smallest number of moles. In this case, H has a number of smallest moles.
The number of C atoms is
and the number of H atoms is
Hence, the empirical formula of the unknown gas is CH.
Since CH is not a stable molecule, then we have to multiply both the number of C and H atoms by 2 in order to become a stable molecule. If you will follow the Octet Rule and Covalent Bonding, the number of electrons surrounding at each C atoms will be equal to eight.
Therefore, the molecular formula of the unknown gas is C2H2 which is acetylene.
"Published in Newark, California, USA"
The following data were obtained in the determination of a HC gas containing 92.3% C by weight at -9°C at various pressure:
| P (atm) | 1 | 2 | 3 |
| ρ (g/mL) | 1.45 | 1.7 | 1.95 |
Identify the gas.
Solution:
The given problem is about finding the molecular formula of a hydrocarbon gas using the principles of empirical and molecular formula. Since the given items are temperature, pressure, and density, then we have to use the Ideal Gas Law in order to get the molecular weight at different pressures and densities.
Let's consider the Ideal Gas Law equation:
but

and the above equation becomes
but

Hence, the Ideal Gas Law equation if the density is given is
where:
MW = molecular weight of unknown gas
ρ = is the density of unknown gas
R = universal gas law constant
T = absolute temperature of unknown gas
P = pressure of unknown gas
Since the units like K, mL, grams, gmoles, and atm are given,then the value of universal gas law constant is

Looking at the given table above, if you get the average of the given pressures and densities, then the value of P is 2 atm and the value of ρ is 1.7 g/mL.
The molecular weight of the unknown gas is
In one mole of unknown gas, the weight of C is
and the weight of H is
The number of moles of C is
and the number of moles of H is
In order to get the number of atoms of C and H, we need to divide them with the smallest number of moles. In this case, H has a number of smallest moles.
The number of C atoms is
and the number of H atoms is
Hence, the empirical formula of the unknown gas is CH.
Since CH is not a stable molecule, then we have to multiply both the number of C and H atoms by 2 in order to become a stable molecule. If you will follow the Octet Rule and Covalent Bonding, the number of electrons surrounding at each C atoms will be equal to eight.
Therefore, the molecular formula of the unknown gas is C2H2 which is acetylene.
Saturday, June 13, 2015
Mole Fraction Problems, 3
Category: Chemical Engineering Math
"Published in Newark, California, USA"
When evacuated or empty, a gas density bulb weighs 29.3215 grams. First, CO2 gas was used to fill the bulb at 40°C and 1.0 atm pressure and it weighed 30.0079 grams. The bulb was then evacuated and filled with the mixture of CO and CO2 under the same temperature and pressure. With this mixture the bulb weighed 29.9332 grams. Determine the % of CO in the mixture.
Solution:
Since the temperature, pressure, and volume are all the same in both cases, then we don't have to use the Ideal Gas Law. We need to use only the principles of Mole Fractions.
Let's consider the first case. If a bulb is filled with CO2, then the weight of CO2 is
and the moles of CO2 is
Since the temperature, pressure, and volume are all the same in both cases, then it follows that
Next, for the second case, if a bulb is filled with a mixture of CO and CO2, then its weight is
Let x be the weight of CO in the mixture so that
Therefore, the weight percent of CO in the mixture is
"Published in Newark, California, USA"
When evacuated or empty, a gas density bulb weighs 29.3215 grams. First, CO2 gas was used to fill the bulb at 40°C and 1.0 atm pressure and it weighed 30.0079 grams. The bulb was then evacuated and filled with the mixture of CO and CO2 under the same temperature and pressure. With this mixture the bulb weighed 29.9332 grams. Determine the % of CO in the mixture.
Solution:
Since the temperature, pressure, and volume are all the same in both cases, then we don't have to use the Ideal Gas Law. We need to use only the principles of Mole Fractions.
Let's consider the first case. If a bulb is filled with CO2, then the weight of CO2 is
and the moles of CO2 is
Since the temperature, pressure, and volume are all the same in both cases, then it follows that
Next, for the second case, if a bulb is filled with a mixture of CO and CO2, then its weight is
Let x be the weight of CO in the mixture so that
Therefore, the weight percent of CO in the mixture is
Subscribe to:
Comments (Atom)