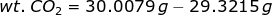Category: Chemical Engineering Math
"Published in Newark, California, USA"
When evacuated or empty, a gas density bulb weighs 29.3215 grams. First, CO2 gas was used to fill the bulb at 40°C and 1.0 atm pressure and it weighed 30.0079 grams. The bulb was then evacuated and filled with the mixture of CO and CO2 under the same temperature and pressure. With this mixture the bulb weighed 29.9332 grams. Determine the % of CO in the mixture.
Solution:
Since the temperature, pressure, and volume are all the same in both cases, then we don't have to use the Ideal Gas Law. We need to use only the principles of Mole Fractions.
Let's consider the first case. If a bulb is filled with CO2, then the weight of CO2 is
and the moles of CO2 is
Since the temperature, pressure, and volume are all the same in both cases, then it follows that
Next, for the second case, if a bulb is filled with a mixture of CO and CO2, then its weight is
Let x be the weight of CO in the mixture so that
Therefore, the weight percent of CO in the mixture is