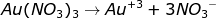Category: Chemical Engineering Math
"Published in Newark, California, USA"
A solvent-water mixture is to be distilled at 95°C. The vapor pressure of the solvent at this temperature is 130 mm Hg and that of water is 640 mm Hg. The solvent is immiscible in water and has a molecular weight of 150. What is the weight of the solvent in kilograms that will be carried over in the distillate with 200 kg of water?
Solution:
From the given word problem, it is about Dalton's Law of Partial Pressure because it involves the mixture of gas vapors which are solvent and water. In this problem, the molecular weight of a solvent is unknown but the partial pressure of solvent and water vapor are given. From the given partial pressures, we can solve for the mole fraction of a solvent vapor as follows
If W is the weight of a solvent and its molecular weight is 150 kg/kgmole, therefore the weight of a solvent that will be carried over in the distillate with 200 kg water is

This website will show the principles of solving Math problems in Arithmetic, Algebra, Plane Geometry, Solid Geometry, Analytic Geometry, Trigonometry, Differential Calculus, Integral Calculus, Statistics, Differential Equations, Physics, Mechanics, Strength of Materials, and Chemical Engineering Math that we are using anywhere in everyday life. This website is also about the derivation of common formulas and equations. (Founded on September 28, 2012 in Newark, California, USA)
Saturday, June 20, 2015
Friday, June 19, 2015
Dalton's Law of Partial Pressure, 3
Category: Chemical Engineering Math
"Published in Newark, California, USA"
If ethanol and methanol are mixed 50/50 by weight at 60°C, and the solution assumed ideal, what is the composition of the vapor above the solution?
Solution:
From the given word problem, it is about Dalton's Law of Partial Pressure because it involves the mixture of gas vapors. Ethanol and methanol are mixed in equal proportion or amount.
Basis: 100 grams of the mixture
Let's consider first the liquid phase. The number of moles of ethanol and methanol in the solution are
Next, consider the gas or vapor phase. From Saturated Vapor Pressure Data, the vapor pressure of pure ethanol at 60°C is 0.461395 atm and for the pure methanol is 0.832997 atm. Hence, the partial pressure of ethanol is
and the partial pressure of methanol is
Therefore, the % ethanol in the vapor is
and the % methanol in the vapor is
"Published in Newark, California, USA"
If ethanol and methanol are mixed 50/50 by weight at 60°C, and the solution assumed ideal, what is the composition of the vapor above the solution?
Solution:
From the given word problem, it is about Dalton's Law of Partial Pressure because it involves the mixture of gas vapors. Ethanol and methanol are mixed in equal proportion or amount.
Basis: 100 grams of the mixture
Let's consider first the liquid phase. The number of moles of ethanol and methanol in the solution are
Next, consider the gas or vapor phase. From Saturated Vapor Pressure Data, the vapor pressure of pure ethanol at 60°C is 0.461395 atm and for the pure methanol is 0.832997 atm. Hence, the partial pressure of ethanol is
and the partial pressure of methanol is
Therefore, the % ethanol in the vapor is
and the % methanol in the vapor is
Thursday, June 18, 2015
Electrochemistry Problems, 3
Category: Chemical Engineering Math
"Published in Newark, California, USA"
An aqueous solution of gold nitrate is electrolyzed with a current of 0.555 amperes until 1.32 g of Au has been deposited on the cathode. If the atomic weight of Au is 197, determine the duration of the electrolysis.
Solution:
During the electrolysis process, gold (III) nitrate solution is ionized completely as follows
With the presence of electric current, gold is deposited on the cathode as follows
By using Faraday's Law of Electrolysis, we can solve for the time or duration of electrolysis of gold as follows
where:
W = weight of a metal formed during electrolysis
EW = equivalence weight of a metal
I = current or rate of flow of electrons
t = time or duration of electrolysis
Therefore, the time or duration of electrolysis of gold is
or
Note: In electroplating industries and jewelry industries, they are using the principles of electrolysis in coating a metal with another metal. A metal to be coated acts as a cathode and another metal used in coating acts as an anode in electrolysis.
"Published in Newark, California, USA"
An aqueous solution of gold nitrate is electrolyzed with a current of 0.555 amperes until 1.32 g of Au has been deposited on the cathode. If the atomic weight of Au is 197, determine the duration of the electrolysis.
Solution:
During the electrolysis process, gold (III) nitrate solution is ionized completely as follows
With the presence of electric current, gold is deposited on the cathode as follows
By using Faraday's Law of Electrolysis, we can solve for the time or duration of electrolysis of gold as follows
where:
W = weight of a metal formed during electrolysis
EW = equivalence weight of a metal
I = current or rate of flow of electrons
t = time or duration of electrolysis
Therefore, the time or duration of electrolysis of gold is
or
Note: In electroplating industries and jewelry industries, they are using the principles of electrolysis in coating a metal with another metal. A metal to be coated acts as a cathode and another metal used in coating acts as an anode in electrolysis.
Subscribe to:
Comments (Atom)