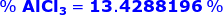Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 1.500 gram sample of impure AlCl3 was dissolved in water and treated with 45.32 mL of 0.1000 M AgNO3 using Mohr Method. Determine its purity.
Solution:
If AlCl3 solution is treated with AgNO3, then the chemical reaction is
From the chemical reaction above, the weight of pure AlCl3 is
Therefore, the percent of AlCl3 in a sample is
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A mixture of LiBr and BaBr2 weighing 800 mg is treated 50.00 mL of 0.1879 M AgNO3 and the excess is found to require 8.76 mL of 0.3179 M KSCN for back titration, using ferric alum as indicator. What is the percentage of BaBr2 in the sample?
Solution:
If AgNO3 is added to the mixture of LiBr and BaBr2, then the chemical reactions are
Let x = be the amount of LiBr in the sample
y = be the amount of BaBr2 in the sample
Since the amount of LiBr and BaBr2 are unknown, then we need to use two equations, two unknowns.
The first working equation is
By gravimetric analysis, the amount of Br in LiBr is
By gravimetric analysis, the amount of Br in BaBr2 is
From the chemical reactions above, the second working equation is
but  Hence, the value of y which is the weight of BaBr2 is
Therefore, the percent of BaBr2 in the samples is
Hence, the value of y which is the weight of BaBr2 is
Therefore, the percent of BaBr2 in the samples is
Category: Chemical Engineering Math
"Published in Newark, California, USA"
What is the molar concentration of AgNO3 solution standardized against 712 mg primary standard NaCl (58.45 g/mole) requiring 23.8 mL of the solution for titration?
Solution:
The
given word problem is about volumetric analysis in which the titration
of a sample and a solution is involved. If AgNO3 solution is added to NaCl solution, then the white precipitate will be formed which is AgCl. in this titration, potassium chromate is added as the indicator. The end point must be a light red color. If all NaCl molecules are used for the titration, then silver chromate will be formed as a reddish color in the solution. The chemical reaction for the titration is
Therefore, the molar concentration of AgNO3 used to standardize NaCl solution is