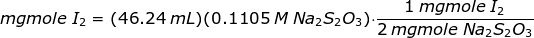Category: Chemical Engineering Math
"Published in Newark, California, USA"
A solution containing 4.48 ppm KMnO4 has a transmittance of 0.576 in a 1.00 cm cell at 520 nm. If the transmittance of an unknown solution is 0.735, what is the concentration of the unknown KMnO4 solution in ppm.
Solution:
The given word problem is about transmittance and absorbance problem in which we can get the concentration of any unknown solutions. Mostly, spectrophotometer is used in getting the concentration of unknown solutions including the blank sample and known sample. By Beer's Law, we can calculate the concentration of any unknown solutions as follows
where:
T = transmittance
A = absorbance
a = molar absorptivity
b = thickness of the cell
c = concentration of solution
If the transmittance of 4.48 ppm KMnO4 is 0.576, then the value of a which is the molar absorptivity is
If the value of transmittance for the unknown KMnO4 solution is 0.735, therefore, its concentration is
This website will show the principles of solving Math problems in Arithmetic, Algebra, Plane Geometry, Solid Geometry, Analytic Geometry, Trigonometry, Differential Calculus, Integral Calculus, Statistics, Differential Equations, Physics, Mechanics, Strength of Materials, and Chemical Engineering Math that we are using anywhere in everyday life. This website is also about the derivation of common formulas and equations. (Founded on September 28, 2012 in Newark, California, USA)
Friday, June 12, 2015
Thursday, June 11, 2015
Oxidation Reduction Reaction Problems, 4
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A sample of iron ore weighing 385.6 mg was dissolved in acid and passed through a Jones Reductor. If the resulting solution required 52.36 mL of 0.01436 M K2Cr2O7 for titration, calculate the % Fe3O4 (231.55 g/mole) in the ore sample.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. If a sample of iron ore is dissolved in an acid and passed through a Jones Reductor, then iron metal is converted into ferrous ion. In order to get the amount of ferrous ion in a solution, K2Cr2O7 solution is used for titration as follows
The amount of ferrous ion or iron in a sample is
The amount of Fe3O4 in a sample of iron ore is
Hence, the weight of Fe3O4 in a sample of iron ore is
Therefore, the weight percent of Fe3O4 in a sample of iron ore is
"Published in Newark, California, USA"
A sample of iron ore weighing 385.6 mg was dissolved in acid and passed through a Jones Reductor. If the resulting solution required 52.36 mL of 0.01436 M K2Cr2O7 for titration, calculate the % Fe3O4 (231.55 g/mole) in the ore sample.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. If a sample of iron ore is dissolved in an acid and passed through a Jones Reductor, then iron metal is converted into ferrous ion. In order to get the amount of ferrous ion in a solution, K2Cr2O7 solution is used for titration as follows
The amount of ferrous ion or iron in a sample is
The amount of Fe3O4 in a sample of iron ore is
Hence, the weight of Fe3O4 in a sample of iron ore is
Therefore, the weight percent of Fe3O4 in a sample of iron ore is
Wednesday, June 10, 2015
Oxidation Reduction Reaction Problems, 3
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 240 mg sample of pyrolusite was treated with excess KI. The iodine liberated required 46.24 mL of 0.1105 M Na2S2O3 solution. Calculate the % MnO2 in the sample.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. The given sample which is pyrolusite contains MnO2 and then treated with excess KI. Na2S2O3 solution is used to titrate the liberated iodine. Sulfuric acid is added as a catalyst as follows
In order to get the amount of iodine liberated, Na2S2O3 solution is used to titrate the iodine crystals as follows
The amount of iodine liberated or iodine crystals is
The amount of MnO2 is
Hence, the weight of MnO2 in a sample of pyrolusite is
Therefore, the weight percent of MnO2 in a sample of pyrolusite is
"Published in Newark, California, USA"
A 240 mg sample of pyrolusite was treated with excess KI. The iodine liberated required 46.24 mL of 0.1105 M Na2S2O3 solution. Calculate the % MnO2 in the sample.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. The given sample which is pyrolusite contains MnO2 and then treated with excess KI. Na2S2O3 solution is used to titrate the liberated iodine. Sulfuric acid is added as a catalyst as follows
In order to get the amount of iodine liberated, Na2S2O3 solution is used to titrate the iodine crystals as follows
The amount of iodine liberated or iodine crystals is
The amount of MnO2 is
Hence, the weight of MnO2 in a sample of pyrolusite is
Therefore, the weight percent of MnO2 in a sample of pyrolusite is
Subscribe to:
Posts (Atom)