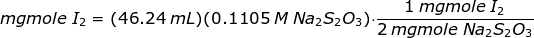Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 240 mg sample of pyrolusite was treated with excess KI. The iodine liberated required 46.24 mL of 0.1105 M Na2S2O3 solution. Calculate the % MnO2 in the sample.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. The given sample which is pyrolusite contains MnO2 and then treated with excess KI. Na2S2O3 solution is used to titrate the liberated iodine. Sulfuric acid is added as a catalyst as follows
In order to get the amount of iodine liberated, Na2S2O3 solution is used to titrate the iodine crystals as follows
The amount of iodine liberated or iodine crystals is
The amount of MnO2 is
Hence, the weight of MnO2 in a sample of pyrolusite is
Therefore, the weight percent of MnO2 in a sample of pyrolusite is