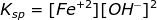Category: Chemical Engineering Math
"Published in Newark, California, USA"
The pH of a saturated solution of Fe(OH)2 is 9.30. Calculate the Ksp of Fe(OH)2.
Solution:
If the pH of Fe(OH)2 is 9.30, then the pOH is
The concentration of hydroxide ion in molarity is
Take the inverse logarithm on both sides of the equation, we have
Since Fe(OH)2 is slightly soluble in water, then the ionization of Fe(OH)2 is
By using mole to mole relationship of the reactants and products in a liter of solution, if
then it follows that
Therefore, the solubility product constant of Fe(OH)2 is