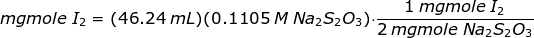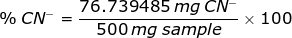Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 240 mg sample of pyrolusite was treated with excess KI. The iodine liberated required 46.24 mL of 0.1105 M Na2S2O3 solution. Calculate the % MnO2 in the sample.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. The given sample which is pyrolusite contains MnO2 and then treated with excess KI. Na2S2O3 solution is used to titrate the liberated iodine. Sulfuric acid is added as a catalyst as follows
In order to get the amount of iodine liberated, Na2S2O3 solution is used to titrate the iodine crystals as follows
The amount of iodine liberated or iodine crystals is
The amount of MnO2 is
Hence, the weight of MnO2 in a sample of pyrolusite is
Therefore, the weight percent of MnO2 in a sample of pyrolusite is
This website will show the principles of solving Math problems in Arithmetic, Algebra, Plane Geometry, Solid Geometry, Analytic Geometry, Trigonometry, Differential Calculus, Integral Calculus, Statistics, Differential Equations, Physics, Mechanics, Strength of Materials, and Chemical Engineering Math that we are using anywhere in everyday life. This website is also about the derivation of common formulas and equations. (Founded on September 28, 2012 in Newark, California, USA)
Showing posts with label Chemical Engineering Math. Show all posts
Showing posts with label Chemical Engineering Math. Show all posts
Wednesday, June 10, 2015
Tuesday, June 9, 2015
Oxidation Reduction Reaction Problems, 2
Category: Chemical Engineering Math
"Published in Vacaville, California, USA"
Calculate the percentage of MnO2 in a 500 mg sample which after the addition of 80.00 mL of 0.1056 M FeSO4 solution required 8.50 mL of 0.0867 M K2Cr2O7?
Solution:
The given word problem is about oxidation reduction reaction or redox in short. A given sample consists of the mixture of impurities and MnO2. FeSO4 solution is used to titrate MnO2 sample and K2Cr2O7 is used for back up titration. Sulfuric acid is added as a catalyst as follows
For back up titration, K2Cr2O7 solution is used to titrate excess FeSO4 solution as follows
The amount of FeSO4 used to titrate MnO2 is
Hence, the weight of MnO2 in a sample is
Therefore, the weight percent of MnO2 in a sample is
"Published in Vacaville, California, USA"
Calculate the percentage of MnO2 in a 500 mg sample which after the addition of 80.00 mL of 0.1056 M FeSO4 solution required 8.50 mL of 0.0867 M K2Cr2O7?
Solution:
The given word problem is about oxidation reduction reaction or redox in short. A given sample consists of the mixture of impurities and MnO2. FeSO4 solution is used to titrate MnO2 sample and K2Cr2O7 is used for back up titration. Sulfuric acid is added as a catalyst as follows
For back up titration, K2Cr2O7 solution is used to titrate excess FeSO4 solution as follows
The amount of FeSO4 used to titrate MnO2 is
Hence, the weight of MnO2 in a sample is
Therefore, the weight percent of MnO2 in a sample is
Monday, June 8, 2015
Oxidation Reduction Reaction Problems
Category: Chemical Engineering Math
"Published in Newark, California, USA"
What is the molarity of a KMnO4 solution standardized against 1.356 gram Na2C2O4 (134 g/mole) requiring 25.1 mL of the solution in acidic medium.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. There's a change of their oxidation numbers in the redox reaction and sulfuric acid is used as a catalyst as follows
Therefore, from the chemical reaction above, the molarity of KMnO4 solution is
"Published in Newark, California, USA"
What is the molarity of a KMnO4 solution standardized against 1.356 gram Na2C2O4 (134 g/mole) requiring 25.1 mL of the solution in acidic medium.
Solution:
The given word problem is about oxidation reduction reaction or redox in short. There's a change of their oxidation numbers in the redox reaction and sulfuric acid is used as a catalyst as follows
Therefore, from the chemical reaction above, the molarity of KMnO4 solution is
Sunday, June 7, 2015
Calcium Hardness Problems, 2
Category: Chemical Engineering Math
"Published in Newark, California, USA"
What volume of 0.0305 M EDTA is needed to titrate the Ca in 178.56 mg of CaCO3?
Solution:
If CaCO3 sample is titrated with EDTA disodium salt solution, then the chemical reaction is
Therefore, from the chemical reaction above, the volume of EDTA disodium salt solution to titrate CaCO3 sample is
"Published in Newark, California, USA"
What volume of 0.0305 M EDTA is needed to titrate the Ca in 178.56 mg of CaCO3?
Solution:
If CaCO3 sample is titrated with EDTA disodium salt solution, then the chemical reaction is
Therefore, from the chemical reaction above, the volume of EDTA disodium salt solution to titrate CaCO3 sample is
Saturday, June 6, 2015
Calcium Hardness Problems
Category: Chemical Engineering Math
"Published in Newark, California, USA"
An EDTA solution prepared from its disodium salt was standardized using 506.3 mg of primary standard CaCO3 and consumed 28.50 mL of the solution. The standard solution was used to determine the hardness of a 2L sample of mineral water, which required 35.57 mL EDTA solution. Express the analysis in terms of ppm CaCO3.
Solution:
If CaCO3 is used to standardized EDTA disodium salt solution, then the chemical reaction is
From the chemical reaction above, we can calculate the concentration of EDTA disodium salt solution as follows
If the standardized EDTA disodium salt solution is used to determine the hardness of mineral water, then the amount of CaCO3 is
Therefore, the amount of CaCO3 in ppm is
"Published in Newark, California, USA"
An EDTA solution prepared from its disodium salt was standardized using 506.3 mg of primary standard CaCO3 and consumed 28.50 mL of the solution. The standard solution was used to determine the hardness of a 2L sample of mineral water, which required 35.57 mL EDTA solution. Express the analysis in terms of ppm CaCO3.
Solution:
If CaCO3 is used to standardized EDTA disodium salt solution, then the chemical reaction is
From the chemical reaction above, we can calculate the concentration of EDTA disodium salt solution as follows
If the standardized EDTA disodium salt solution is used to determine the hardness of mineral water, then the amount of CaCO3 is
Therefore, the amount of CaCO3 in ppm is
Friday, June 5, 2015
Volumetric Analysis Problems, 10
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 750.25 mg alloy of nickel was dissolved and treated to remove the impurities. The ammonium solution was treated with 50 mL of 0.1075 M KCN and the excess cyanide required 2.25 mL of 0.00925 M AgNO3. Determine the % Ni in the alloy.
Solution:
If nickel is dissolved in ammonium solution, then nickel is converted into nickel ion in order to remove the impurities. If KCN solution is added to nickel solution, then the chemical reaction is
From the chemical reaction above, we can solve for the weight of nickel as follows
Therefore, the percent of nickel in the alloy is
"Published in Newark, California, USA"
A 750.25 mg alloy of nickel was dissolved and treated to remove the impurities. The ammonium solution was treated with 50 mL of 0.1075 M KCN and the excess cyanide required 2.25 mL of 0.00925 M AgNO3. Determine the % Ni in the alloy.
Solution:
If nickel is dissolved in ammonium solution, then nickel is converted into nickel ion in order to remove the impurities. If KCN solution is added to nickel solution, then the chemical reaction is
From the chemical reaction above, we can solve for the weight of nickel as follows
Therefore, the percent of nickel in the alloy is
Thursday, June 4, 2015
Volumetric Analysis Problems, 9
Category: Chemical Engineering Math
"Published in Newark, California, USA"
A 500 mg sample containing NaCN required 23.50 mL of 0.1255 M AgNO3 to obtain a permanent faint turbidity. Express the result of this analysis as %CN-.
Solution:
If AgNO3 is added to NaCN, then the chemical reaction is
From the chemical reaction above, the weight of CN- is
Therefore, the percent of CN- in a sample is
"Published in Newark, California, USA"
A 500 mg sample containing NaCN required 23.50 mL of 0.1255 M AgNO3 to obtain a permanent faint turbidity. Express the result of this analysis as %CN-.
Solution:
If AgNO3 is added to NaCN, then the chemical reaction is
From the chemical reaction above, the weight of CN- is
Therefore, the percent of CN- in a sample is
Subscribe to:
Posts (Atom)